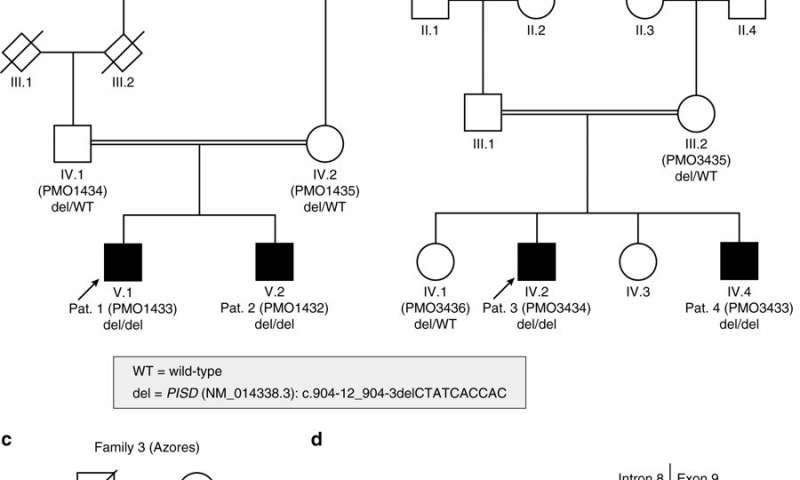
Three unrelated families on three continents (from continental Portugal, the United States and Brazil), all with healthy ancestors, had children with a very rare multi-organ condition that causes early-onset retinal degeneration, sensorineural hearing loss, microcephaly, intellectual disability, and skeletal dysplasia with scoliosis and short stature.
Genetic analysis by a team led by Carlo Rivolta, group head for genetic ophthalmology at IOB, and Prof. Andrea Superti-Furga, head of the division of genetic medicine at Lausanne University Hospital, has revealed that the disease traces back to a common founder variant, possibly originating from a healthy carrier of the mutation living in Portugal approximately 125 years ago.
“In 1986, the ophthalmologist Ruth Liberfarb and her co-workers first described the condition in a patient originating from the Azores but residing in the U.S. at time of diagnosis. We could show in our study that the molecular cause of disease is the same in all patients. Therefore, Andrea Superti-Furga had the idea we could suggest calling it Liberfarb syndrome,” says Carlo Rivolta.
Hope for future therapies
The research team is particularly pleased that their work did not only document the likely migration of a rare genetic mutation from Portugal to two continents, but also highlights the link between phospholipid metabolism and bone formation, sensory defects, and cerebral development, while raising the possibility of therapeutic phospholipid replacement for future patients.
Liberfarb syndrome
Liberfarb syndrome is caused by a homozygous recessive defect in phosphatidylserine decarboxylase (PISD). PISD is particularly enriched in mitochondrial membranes and required for viability. Affected individuals examined in this study shared a 3.36 Mb region of autozygosity on chromosome 22q12.2, including a 10-bp deletion (NM_014338.3:c.904-12_904-3delCTATCACCAC), immediately upstream of the last exon of the PISD gene. Sequencing ofPISDfrom paraffin-embedded tissue from the 1986 case revealed the identical homozygous variant, which likely does not completely abolish phosphatidylserine decarboxylase activity, but results in a severe disease. Individuals carrying other mutations in PISD show quite divergent clinical phenotypes, possibly related to the severity of the variants detected. They are ranging from apparently isolated skeletal dysplasia to multi-systemic conditions affecting brain, ear, eye, connective tissue, and bone.

Leave a Comment